Question Answers
Surface Tension
1. Soap bubbles are almost perfect spheres. Why?
Soap bubbles have large surface area and negligible mass. Thus, the effect of gravitational force on the bubble is almost nil. Then the surface tension of the thin layer of soap tends to minimize its surface area. Hence, the bubbles attain a perfect sphere.
2. Why a soap solution a better cleansing agent than ordinary water?
A soap solution has lower surface tension than ordinary water. Due to lower surface tension, the dirt and dust particles easily get mixed with soap solution and washed away. For the ordinary water, the surface tension is high and thus the dust particles do not get mixed with water easily. So, a soap solution is a better cleansing agent than ordinary water.
3. Antiseptic used in cuts and human flesh have low surface tension. Why?
Antiseptics has low surface tension so that it spreads along the wounds and cuts uniformly and cover it. Hence, it reduces the exposure of wounds and cuts to the germs and prevents from infection.
4. How do the leaves of tree get water from the ground?
The stem of the tree consist of a lot of fine pores through them which function similar to capillary tubes. The roots of the tree are deep in contact with the water resources below the ground. The water rises through these pores as given by relation in the capillary tube, $$h=\frac{2T cos \theta}{r \rho g}$$ Since, the radii of the fine pores is very small, the rise of water is very high.
5. What are cohesive and adhesive forces?
Cohesive force is the force of attraction between the molecules of the same substance. Adhesive force is the force of attraction between the molecules of the different substance.
6. What will happen if a capillary tube of insufficient height is dipped in water?
When, the capillary tube of insufficient height is dipped in water, the water rises up to the top of the tube and spread itself on the material of the tube without overflowing. The angle of contact becomes 900 at the top and hence $$h=\frac{2T cos \theta}{r \rho g} = 0$$ So, there is no further rise of water.
7. Why are small liquid drops spherical while large drops are flat?
For a small liquid drop, mass is less and thus the downward force of gravity is less. The surface molecules experiences surface tension forces and thus attracted inward. Thus the liquid surface tends to have minimum surface area. For a given volume, the sphere has a minimum surface area. So, small liquid drops are spherical in shape.
When the drops are large, mass is large, and the force of gravity will be high. The molecules are attracted due to surface tension as inward as possible and also receive a downward force due to force of gravity. So, some molecules slide downwards, giving the whole drop a flattened shape.
8. The tip of the nib of a pen is split. Why?
The split in the tip of the nib of a pen with a small separation of a capillary tube. The ink in the pen rises to the nib through the split upto the tip. This makes writing possible.
9. Small particles of computer dance on the surface of water. Why?
Water has high surface tension. When small particles camphor is placed on it, the camphor doesnot wet with water. The force of cohesion between the water molecules is high than the force of adhesion between the water molecules and camphor. So, water molecules are attracted towards each other giving an opposite force on the camphor in contact with water molecules. So, the camphor particle moves backward randomly and seems to be dancing.
10. Hot soup is more tasty than a cold one. Why?
The surface tension of hot soup is less than a cold one. This makes the soup spread better on the tongue increasing its access to the taste buds. So, the taste is good and complete.
11. We use towels to dry our body after talking a shower. Why?
Towels consists of very thin fibres interwoven on each other. This causes the formation of a lot of thin channels in between them which act as the capillary tubes. So, when a towel is placed over wet surface, water climbs into these channels according to the relation, $$h=\frac{2T cos \theta}{r \rho g}$$ This takes water away from the skin and leaves the skin dry. However, this leaves the towel wet.
12. Hairs of a brush spread out when it is dipped in water and cling together as soon as it is taken out of water. Explain.
: When the hair brush is in water, there are water molecules all around its hair. Consequently, there is no free surface and the effect of surface tension is absents. But when the brush is taken out from water, the water molecules in between the hairs are attracted inwards due to force of surface tension. So, they cling together as soon as it is taken out of water.
13. Why are small drops of mercury spherical and bigger drops oval in shape?
A drop of liquid experiences two forces: force of surface tension and force of gravity. Due to the force of surface tension, the liquid minimizes its surface area and tends to have spherical shape. Due to the force of gravity, the liquid tends to occupy the lowest center of gravity and hence tends to become flat.
In case of small drops of mercury, the force of gravity is negligible. So, the drops gains spherical shape due to the effect of surface tension. But in case of large drops, both the force of gravity and force of surface tension acts on the mercury drops. As a result, the drops gains oval shape.
14. Why does mercury inside the capillary tube made of glass depress when dipped in a reservoir of mercury?
In case of mercury glass, the adhesive force is less than cohesive force between mercury molecules. So, the mercury molecules in the capillary tube are more attracted downwards. As a result, the mercury depresses in the capillary tube.
15. It is observed that the surface of a liquid in a capillary tube dipped in it is either convex or concave. What may be the reason? Explain.
When a capillary tube is dipped in a liquid, the surface of a liquid in the tube is curved. The nature of the curved surface depends on the relative magnitudes of the cohesive forces and adhesive forces acting at the point of contact.
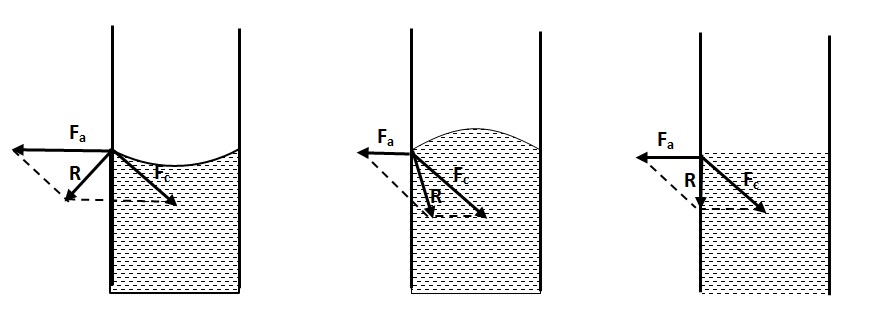
When the resultant adhesive force Fa between the neighbor glass and water molecules is greater than the resultant cohesive force Fc between the neighbor water molecules, i.e. Fa > Fc, their resultant R is directed outward and as a result the liquid molecules stick to the wall of the tube and the surface will be concave. But when Fa < Fc, their resultant will be directed inward and as a result the liquid molecules tend to move away from the tube wall and the surface will be convex. If the two forces are equal, their resultant will directed downward and the surface will be plane.
16. Why mercury does not wet the glass tube?
In case of mercury-glass, the adhesive force is less than cohesive force between molecules. So, the mercury molecules are more attracted towards other mercury molecules than the glass surface. As a result, the mercury does not wet the glass tube.
17. On what factors does the surface tension of a liquid depend? Explain.
Surface tension of a liquid depends upon the following factors: (i) Temperature (ii) Impurities in the liquid (iii) Solutes in the liquid